
-
Affects over 425 million worldwide
425
million -
1 in 5 adults have at least mild OSA
20%
-
25% of middle-aged men have OSA
25%
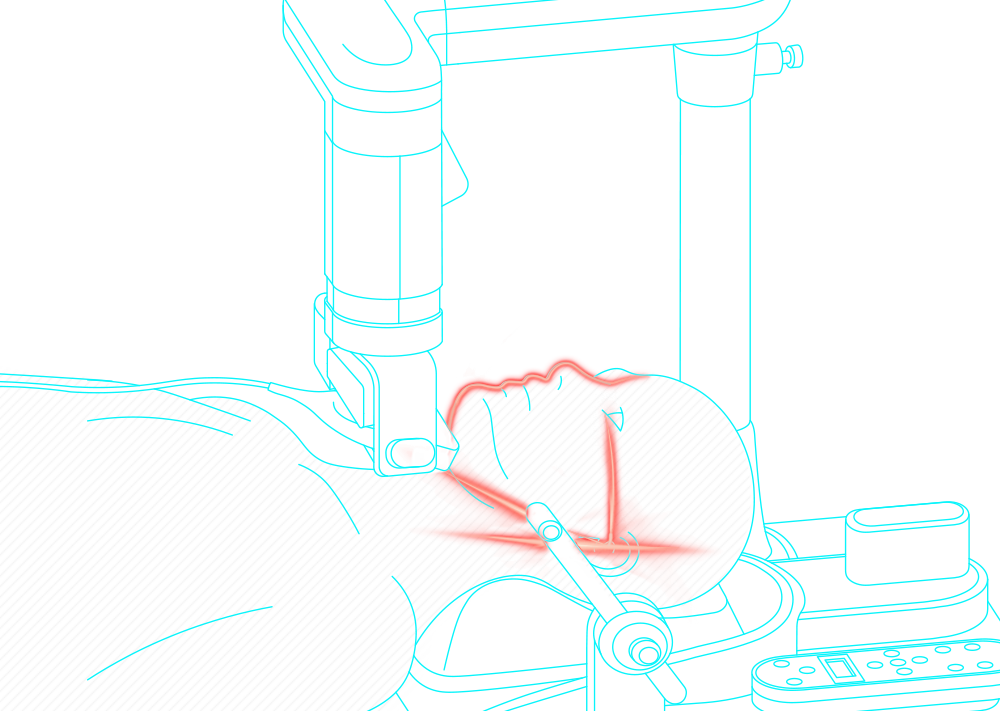
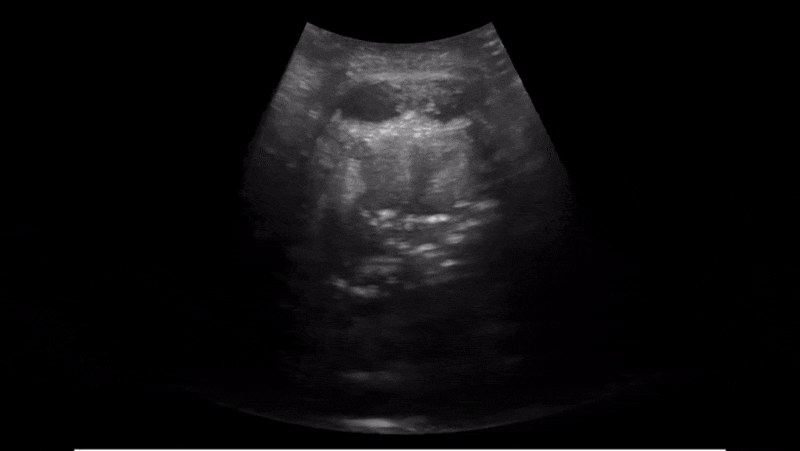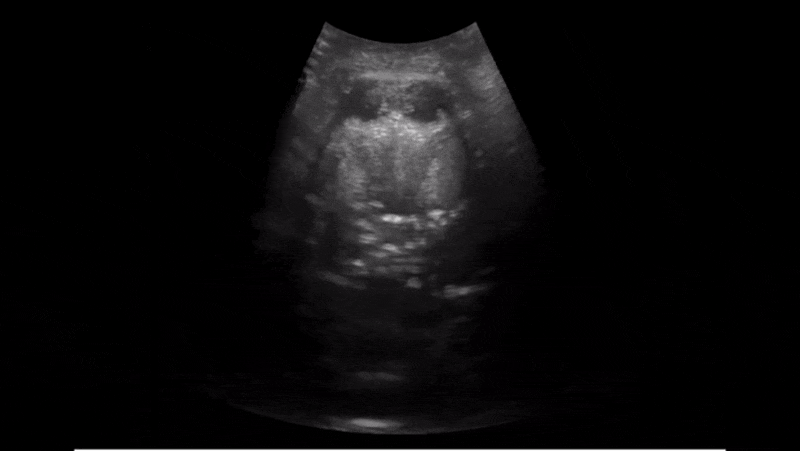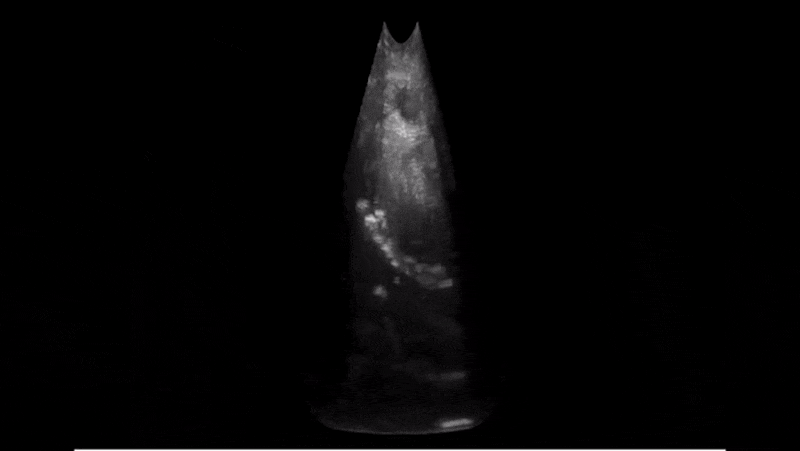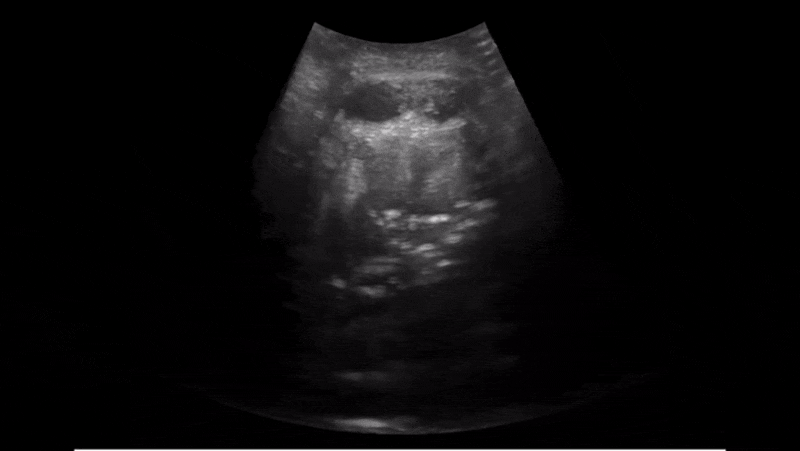
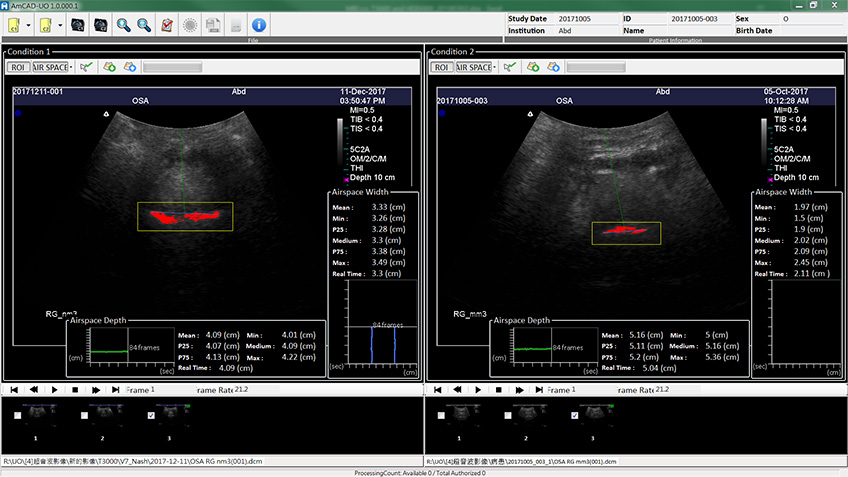
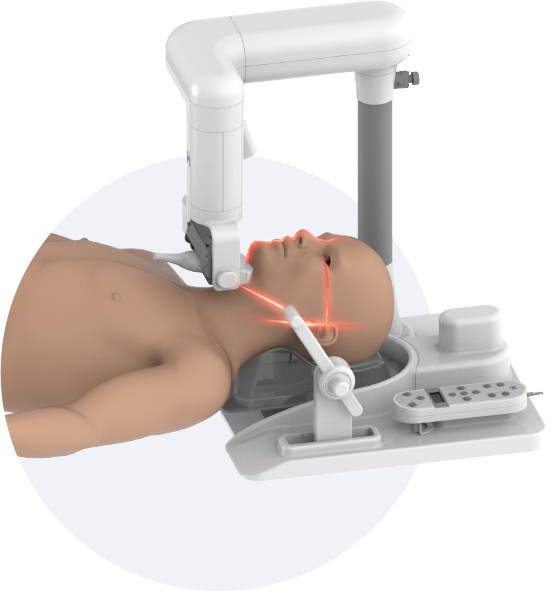
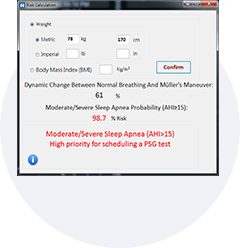
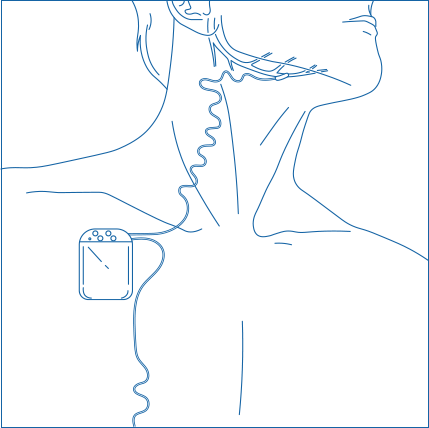
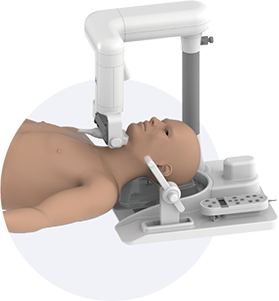
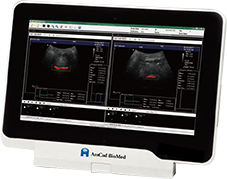
Obstructive sleep apnea is a potentially serious sleep disorder. It causes breathing to repeatedly stop and start during sleep.







 are the trademarks owned by AmCad in Taiwan and/or in other countries. Unauthorized use of the AmCAD trademarks is prohibited unless specifically authorized.
are the trademarks owned by AmCad in Taiwan and/or in other countries. Unauthorized use of the AmCAD trademarks is prohibited unless specifically authorized.